LUGANO-GENEVA, Switzerland – Further evidence that immunotherapy provides long-term survival benefit for patients with lung cancer was presented today at ELCC 2018 (European Lung Cancer Congress) in Geneva, Switzerland.
Researchers presented the three-year survival results of the randomised phase 2 POPLAR trial in second line, (1) which is the longest follow-up reported to date with anti-programmed death ligand 1 (PD-L1) immunotherapy in patients with previously treated, advanced non-small-cell lung cancer (NSCLC). The trial randomised 287 patients from 61 sites across 13 countries with advanced NSCLC to the anti-PD-L1 antibody atezolizumab or docetaxel (chemotherapy).
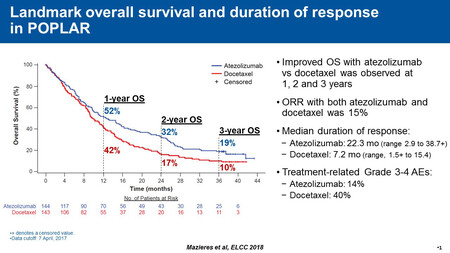
Overall survival was significantly higher with atezolizumab at two and three years compared with docetaxel. Nearly one-third of patients (32.2%) in the atezolizumab treatment group were alive at two years compared with 16.6% in the docetaxel group. Additionally, at three years, almost twice as many patients (18.7%) were alive in the atezolizumab group compared to the docetaxel group (10.0%). The long-term overall survival benefit with atezolizumab over docetaxel was observed across histology (squamous and non-squamous) and regardless of PD-L1 expression. Even patients with PD-L1 expression in less than 1% of tumour cells and less than 1% of immune cells had a promising rate of long-term survival.
The median duration of response was three times longer with atezolizumab (22.3 months) compared to docetaxel (7.2 months). Atezolizumab led to fewer adverse events than docetaxel.
Lead author Dr Julien Mazières of Toulouse University Hospital, Toulouse, France, said: “Nearly one in five patients treated with atezolizumab was alive at three years. This places atezolizumab among the drugs with the highest landmark overall survival in previously treated lung cancer patients.”
“The fact that all subgroups of patients benefitted to a similar degree is good in the sense that atezolizumab can be tried in all advanced NSCLC patients,” he continued. “On the other hand, it means that we cannot predict which patients are most likely to live for three years. We need to find biomarkers to help us identify the long-term survivors with the drug.”
Mazières said the drug was well tolerated which meant that patients can keep taking it for several years. He said: “Some of my patients who were in the atezolizumab treatment group are now long-term survivors with lung cancer. They are not cured, but they have survived, have a good quality of life, and have returned to work. With immunotherapy we now have a new type of patient: long-term survivors with lung cancer who can go back to a normal life.”
Commenting on the study, Prof Solange Peters, Head of Medical Oncology, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, ESMO President-Elect said: “Before immunotherapy, the long-term survival of non-small-cell lung cancer patients was close to 0%. POPLAR supports the concept that long-term survival is possible with immunotherapy. The three-year survival results of POPLAR are consistent with the three- and five-year survival with the anti-PD-1 antibodies pembrolizumab and nivolumab, respectively, in phase 1 trials. These latest results are exciting because unlike the previous two trials, POPLAR was a large, randomised trial and provides convincing proof that long-term survival now exists in lung cancer.”
Peters said there was now a strong argument that every patient with advanced NSCLC should receive immunotherapy. She said: “In the nivolumab phase 1 trial 15% of patients were alive at five years, which in cancer is usually considered being cured. We should offer all patients this one in six chance of five-year survival. However, this poses a financial challenge for healthcare systems.”
To make this strategy sustainable, Peters said a method was needed to identify the patients who will not benefit from immunotherapy. She said: “That would enable us to treat only the patients with a high chance of long-term survival with immunotherapy. POPLAR shows that PD-L1 is not a useful biomarker to exclude patients from immunotherapy, since some patients with very low expression had an overall survival benefit. Rather than a single biomarker, I think it will be a signature of many biomarkers including tumour mutation burden that identifies the patients who should not be treated.”
Peters said trials are needed to assess the ability of a combination of biomarkers to predict which patients with advanced NSCLC do, and do not, survive long-term with immunotherapy: “These trials should be conducted in patients with similar characteristics to the long-term survivors in the phase 1 and 2 trials with atezolizumab, pembrolizumab, and nivolumab. So the first step will be to describe these patients in terms of demographics, smoking history, tumour mutation burden, expression of immune genes, and PD-L1 expression. Focusing future studies on these patients will help us to discover a biomarker signature for use in clinical practice.”
Notes to Editors
Please make sure to use the official name of the meeting in your reports: ELCC 2018
Official Congress hashtag: #ELCC2018
References
- Abstract 136PD_PR "3-year survival and duration of response in randomized phase II study of atezolizumab (atezo) vs docetaxel (doc) in 2L+ NSCLC (POPLAR)": presented by Julien Mazières during the Poster Discussion session "Immunotherapy and next-generation TKIs: from second to frontline treatment" on Thursday, 12 April, 07:45 to 09:00 (CEST) in Room A.
Journal of Thoracic Oncology, Volume 13, Issue 4, Supplement, April 2018.
About the European Society for Medical Oncology (ESMO)
ESMO is the leading professional organisation for medical oncology. With 18,000 members representing oncology professionals from over 150 countries worldwide, ESMO is the society of reference for oncology education and information. We are committed to supporting our members to develop and advance in a fast-evolving professional environment.
ESMO’s educational resources support an integrated, multi-professional approach to cancer care. We have European roots and a global reach: we welcome oncology professionals from around the world and we seek to erase boundaries in cancer care as we pursue our mission across oncology, worldwide.
About the International Association for the Study of Lung Cancer (IASLC)
The International Association for the Study of Lung Cancer (IASLC) is the only global organisation dedicated solely to the study of lung cancer and other thoracic malignancies. Founded in 1974, the association's membership includes more than 6,500 lung cancer specialists across all disciplines in over 100 countries, forming a global network working together to conquer lung and thoracic cancers worldwide. The association also publishes the Journal of Thoracic Oncology, the primary educational and informational publication for topics relevant to the prevention, detection, diagnosis and treatment of all thoracic malignancies. Visit www.iaslc.org for more information and follow us on Twitter @IASLC.
136PD_PR: 3-year survival and duration of response in randomized phase II study of atezolizumab (atezo) vs docetaxel (doc) in 2L+ NSCLC (POPLAR)
J. Mazières1, K. Park2, C. Lewanski3, S. Gadgeel4, L. Fehrenbacher5, A. Rittmeyer6, J.Y Han7, A. Artal-Cortes8, F. Braiteh9, M. Gandhi10, W. Yu10, C. Matheny10, P. He10, A. Sandler10, M. Ballinger10, J. Vansteenkiste11
1Thoracic Oncology, Toulouse University Hospital, Toulouse, France, 2Samsung Medical Center Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, 3Charing Cross Hospital, London, UK, 4University of Michigan, Ann Arbor, MI, USA, 5Kaiser Permanente Medical Center, Vallejo, CA, USA, 6Lungenfachklinik Immenhausen, Immenhausen, Germany, 7National Cancer Center, Goyang, Republic of Korea, 8Hospital Universitario Miguel Servet, Zaragoza, Spain, 9Comprehensive Cancer Centers of Nevada, Las Vegas, NV, USA, 10Genentech, Inc., South San Francisco, CA, USA, 11University Hospitals KU Leuven, Leuven, Belgium
Background: Atezo (anti–PD-L1) has demonstrated OS benefit over doc in a randomized Phase II study, POPLAR, in patients with advanced NSCLC. This benefit has been confirmed in the randomized Phase III study OAK (Rittmeyer, 2017). The 3-year survival analysis of the POPLAR study presented here describes the longest survival follow-up reported to date of an all-comer randomized PD-L1/PD-1 immunotherapy trial in 2L+ NSCLC.
Methods: Patients were randomized 1:1 to receive atezo (1200 mg) or doc (75 mg/m2) IV q3w. Tumors were prospectively evaluated for tumor cell (TC) or tumor-infiltrating immune cell (IC) PD-L1 expression using the VENTANA SP142 IHC assay. Landmark OS was estimated using the Kaplan-Meier method. Data cutoff, April 7, 2017; minimum follow-up, 3 years.
Results: The 2-year and 3-year survival with atezo vs doc were 32.2% vs 16.6% and 18.7% vs 10.0%, respectively. The long-term OS benefit of atezo vs doc was observed across histology and PD-L1 expression subgroups (Table). While the TC3 or IC3 subgroup derived the greatest OS benefit, the TC0 and IC0 subgroup also had improved long-term OS with atezo vs doc. The ITT ORR was 15% in both atezo and doc arms, but the median duration of response was 3 times longer with atezo (22.3 months [95% CI: 11.6, 31.1] vs 7.2 months [95% CI: 5.8, 12.2] with doc). Seven of the 11 doc-arm 3-year survivors received subsequent non-protocol therapy with anti–PD-L1/PD-1 agents. Atezo had a favorable safety profile compared with doc that was consistent with previous reports.
Conclusions: Atezo demonstrates superior 2-year and 3-year OS benefit compared with doc, and this benefit is observed across histology and PD-L1 expression subgroups (including TC0 and IC0). Atezo is well tolerated, and responses are highly durable. These results are consistent with long-term OS results from OAK (Satouchi, WCLC 2017).
Table: Landmark OS in the ITT, PD-L1 expression, and histology subgroups in POPLAR
a For descriptive purpose only. TC3 or IC3 = PD-L1 ≥ 50% TC or 10% IC; TC2/3 or IC2/3 = PD-L1 ≥ 5% TC or IC; TC1/2/3 or IC1/2/3 = PD-L1 ≥ 1% TC or IC; TC0 and IC0 = PD-L1 < 1% TC and IC. NCT01903993.
Clinical trial identification: NCT01903993
Legal entity responsible for the study: F. Hoffmann-La Roche Ltd/Genentech, Inc.
Funding: F. Hoffmann-La Roche Ltd/Genentech, Inc.
Disclosure: K. Park: Consulting/Advisory Role: Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Clovis Oncology, Lilly, Hanmi, Kyowa Hakko Kirin, Novartis, Ono Pharmaceutical, Roche. Speakers Bureau: Boehringer Ingelheim, Research Funding: AstraZeneca. C. Lewanski: Consultant: MSD, Roche, AstraZeneca. S. Gadgeel: Speaker's bureau- Astra-Zeneca, Genentech/Roche Advisory Boards: Astra-Zeneca, Ariad, Pfizer, Bristol Myers Squibb and Genentech/Roche. A. Rittmeyer: Grants as an advisor or speaker by: Astra Zeneca, BMS, Boehringer Ingelheim, Eli Lilly, Pfizer and Roche Genentech. A. Artal-Cortes: Advisory boards: Roche, BMS, MSD Travel fees: Roche. F. Braiteh: Speaking and consulting fees received From Genentech. M. Gandhi: Genentech employee and Roche stock. W. Yu: Genentech employee and Roche stock. C. Matheny: Genentech employee and Roche stock, Roche/Genentech travel, accommodations, expenses patents, royalties or other intellectual property: Stanford University (patient with Stanford, do not currently receive royalties or have other intellectual property). P. He: Genentech employee and Roche stock. A. Sandler: Genentech employee; Roche and Amgen stock, Husband has stocks for Allergan and Gilead. M. Ballinger: Genentech employee and Roche stock. All other authors have declared no conflicts of interest.
This press release contains information provided by the authors of the highlighted abstracts and reflects the content of those abstracts. It does not necessarily reflect the views or opinions of ESMO or IASLC who cannot be held responsible for the accuracy of the data. Commentators quoted in the press release are required to comply with the ESMO Declaration of Interests policy and the ESMO Code of Conduct.